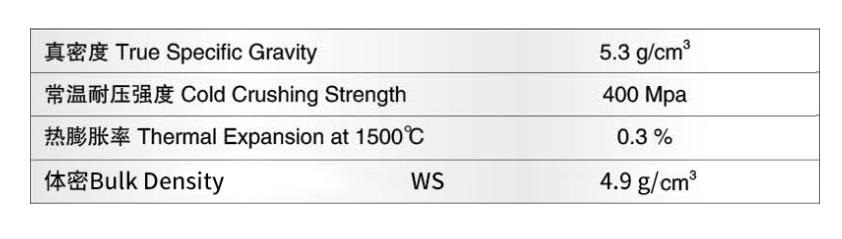
The basic properties of zirconia bricks
1. The basic properties of zirconia:
Zirconium dioxide is a high melting point metal oxide with a molecular formula of ZrO2, a relative molecular mass of 123.22, a melting point of 2715°C, a softening point between 2390 and 2500°C, a boiling point of about 4300°C, Mohs hardness of 7, and a density of 5.65. ~6.27g/cm3, the average linear expansion coefficient at 20~1000℃ is 10×10-6/℃, and the thermal conductivity at 1000℃ is 2.30W/(m·K).
Pure zirconia is a white powder, slightly yellow or gray when it contains impurities, and contains hafnium dioxide impurities. Because zirconium dioxide has the characteristics of wear resistance, high-temperature resistance, corrosion resistance, non-conductivity, non-magnetic conductivity, etc., and has a linear expansion coefficient similar to that of metal, it is the only oxide among the oxides that have martensitic transformation with steel and other materials. Alloy-like materials make zirconia a number of important uses.
Zirconia has three different crystal structures: low-temperature phase, medium temperature phase, and high-temperature phase. Zirconia at high temperature belongs to the cubic fluorite structure, the intermediate temperature phase is the tetragonal zirconia crystal structure and the low-temperature phase monoclinic zirconia crystal structure. The linear expansion coefficients of the three crystal forms are different, the monoclinic zirconia is the smallest, the tetragonal zirconia is the second, and the cubic zirconia is the largest. This is because the thermal properties of the material, such as heat capacity, thermal conductivity, and thermal expansion, are all related to the thermal vibration of atoms, that is, directly dependent on the vibration of the lattice.
Under the premise of only considering the composition of the material phase, for zirconia, because the lattice structure of the cubic phase is the simplest, the thermal vibration of the atoms is relatively easy, while the monoclinic phase structure is the most complex, and the thermal vibration of the atoms is relatively difficult.
2. Crystal transformation of zirconia:
The three crystal forms of zirconia have a reversible phase transformation process with the change of temperature, in which the phase transformation of the tetragonal monoclinic phase changes to the martensitic phase transformation, which was first pointed out by G.M.Wolten, and this phase transformation is used in the research of zirconia materials. of particular importance. The transformation from monoclinic to tetragonal crystal form is reversible with 7% volume shrinkage. That is, it shrinks when it heats up and expands when it cools down.
However, the monoclinic tetragonal forward transformation starts at 1170 °C, while the reverse transformation is between 1000 and 850 °C. Due to the difficulty in the formation of monoclinic crystal nuclei, temperature hysteresis occurs in the crystal phase transformation. ZrO2 is monoclinic when it is not higher than 1000 °C, and tetragonal when it is higher than 1000 °C. Observing the experimental diagram of the thermal expansion performance of different zirconias, although the linear expansion coefficient of pure monoclinic zirconia is small, its linear expansion has significant anisotropy, and there is also the problem of phase transition.
Although the axial expansion of tetragonal zirconia is different, the difference is not large, and it is similar to a linear relationship, so the expansion uniformity is better, and no sudden change and brittle crack will occur; the thermal expansion of cubic ZrO2 is carried out along the uniaxial, and with increases with temperature; the thermal expansion of partially stabilized ZrO2 is intermediate between monoclinic and tetragonal phases.
3. Stabilization of zirconia: ZrO2 stabilization is to rebuild the tetragonal lattice into a cubic lattice that is stable at any temperature. At the same time as the lattice is rebuilt, a solid solution composed of stabilizer and ZrO2 is produced. These solid solutions are A substitutional solid solution with limited solubility, which is formed from certain oxides with cationic radii similar to the Z4+ ionic radius (0.087 nm).
The more commonly used ones are Y2O3, MgO, CeO2, and CaO. At present, the stabilization mechanism is not very clear. It is generally explained that the cations of stabilizers such as Y3+, Mg2+, Ce4+, and Ca2+ have a certain solubility in ZrO2, and can replace Zr in it to form a replacement solid solution.
The essence of zirconia stabilized by different stabilizers is roughly the same, but the performance after they are stabilized is not the same, and the performance obtained by the same stabilizer with different additions is also quite different. Therefore, the selection of the appropriate stabilizer and the addition amount is crucial to the performance of the zirconia material.
Zirconium brick has the characteristics of good corrosion resistance, low foaming rate, and firm rate, almost no pollution to glass liquid, suitable for various glass furnaces, especially high-quality glass furnaces and special glass furnaces, Mainly used for glass kiln walls, liquid holes, electrode bricks, kiln sills, and other parts.
-

Thermal storage alumina balls
The Thermal storage alumina ballsis made of industrial alumina and refractory kaolin as the main raw materials through scientific formula, forming and high-temperature calcination.Thermal storage alumina ballss are divid··· -

Anti-stripping high alumina brick
Use description of Anti-stripping high alumina brick1. Anti-stripping high alumina brick has a good application in low temperature parts such as large and medium-sized cement precalciner, kiln smoke chamber, indoor decom··· -

Anti-stripping high alumina bricks
Anti-stripping high alumina bricks are made of high alumina bauxite clinker, mullite, kyanite, zircon sand, and binder after granulating and powdering processes, mixed in a certain proportion, pressed into shape, and fir··· -

silica hot repair refractory
Performance index of silica hot repair refractoryThe material is a kind of plastic unshaped refractory material, its main component is SiO2, it is made of special clinker and various binders and additives, and it is proc···

